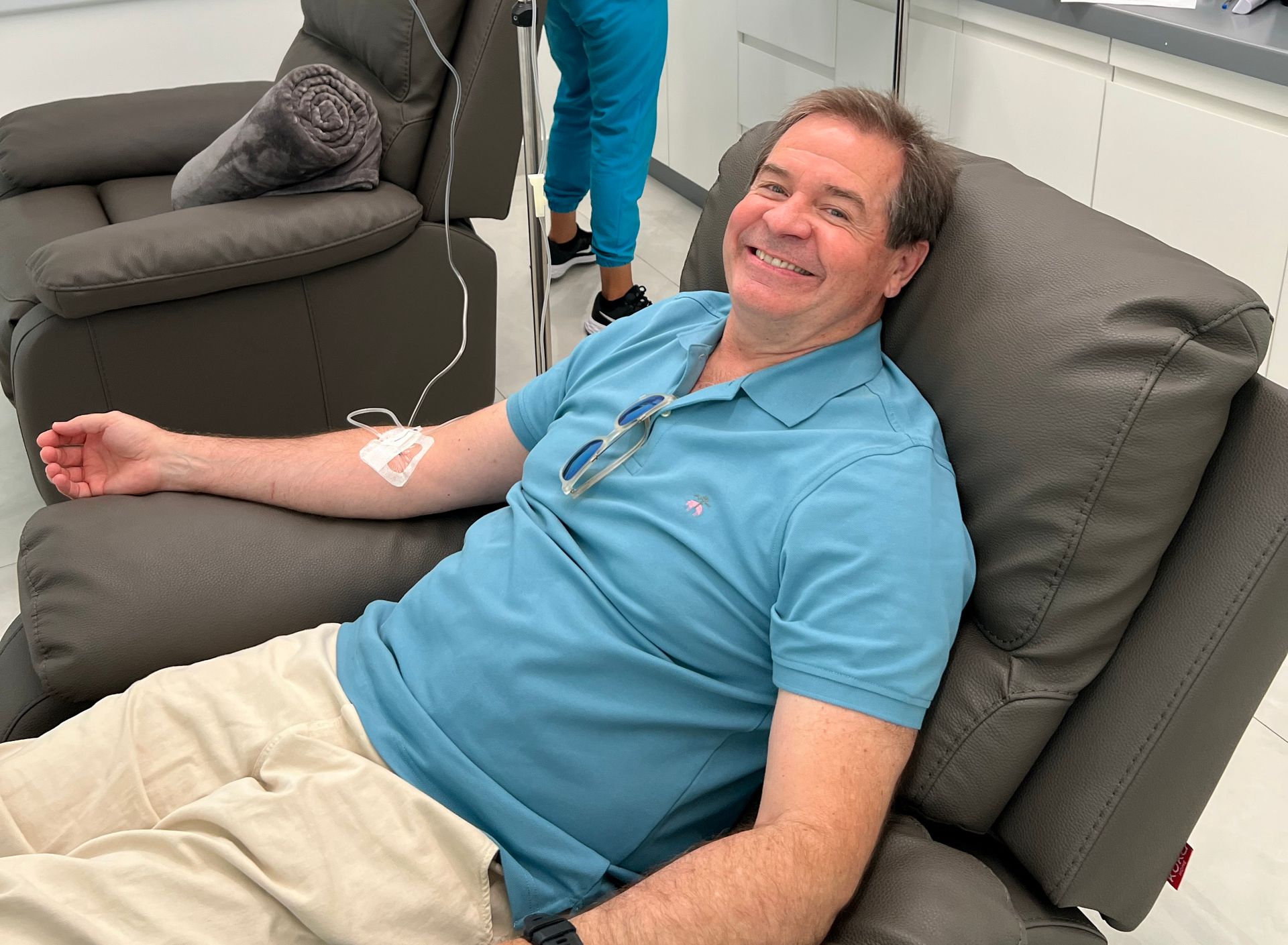
CLL Patient
Our CLL patient a 64-year-old med tech veteran, was diagnosed with Chronic Lymphocytic Leukemia in 2021. Under the care of Mayo Clinic in Jacksonville, Florida, he initially responded to treatment, but the outlook changed dramatically when a mutation was discovered that shifted his prognosis from favorable to poor. Despite three years of targeted therapy—including treatment with Calquence, a second-generation BTK inhibitor—he never achieved remission. By late 2023, his bone marrow still showed 20% tumor cell infiltration, and he was suffering from persistent fatigue, body pain, cognitive fog, and immune dysfunction.
On December 5, 2023, he became the first patient to receive NKore’s investigational cell therapy, NK101—a single infusion of approximately 191 million supercharged NK cells. Post-treatment bloodwork indicated a rapid, favorable immune response with no adverse safety events. Most notably, his 90-day follow-up revealed early signs of partial remission after just one dose. Objective markers confirmed that NK101 had successfully targeted and eliminated tumor cells in the blood and bone marrow, reduced disease burden, and partially restored immune system function. These early clinical results corresponds with Dr. Anahid Jewett’s preclinical findings and offer compelling evidence of NK101’s therapeutic potential.
A follow-up bone marrow aspirate performed on Day 58 post-infusion showed a 75% reduction in tumor cell infiltration, with only 5% minimal residual disease remaining—down from 20% prior to NK101 administration.
Our patient's experience was not just clinical—it was transformational. His story is not only a
testament to the potential of NK cell therapy—it’s a reminder of what’s possible when science meets purpose.