Immunotherapy
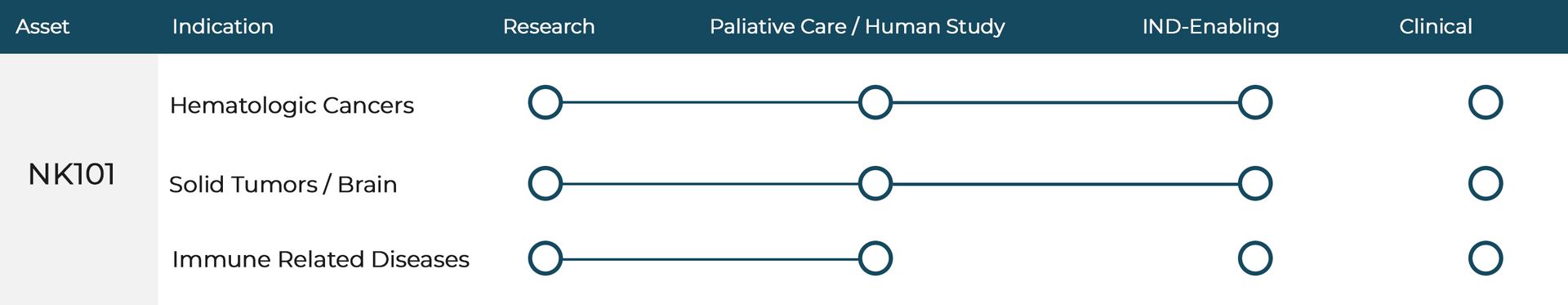
NK101: Supercharged NK Cell Therapy
Pathway to Market
At NKore BioTherapeutics, our pathway to market is strategically designed to accelerate patient access to our non-toxic immunotherapy. We are currently generating real-world data from a human study in Cancun, Mexico, where patients are being treated within a controlled clinical framework. The promising results from this study are informing our U.S. regulatory strategy and serving as the foundation for our pre-IND submission to the FDA. We are working closely with experienced regulatory advisors and plan to file our Investigational New Drug (IND) application by the end of 2025, with the goal of initiating U.S.-based clinical trials shortly thereafter.
Supercharging The Innate Immune System
Just as T-cells are the core of the adaptive immune system, Natural Killer (NK) cells are the core of the innate immune system; the immune system you are born with. As such, NK cells are unique in their ability to identify and destroy cancer stem and stem-like cells and trigger the differentiation of aggressive undifferentiated tumors to make them more susceptible to other forms of cancer treatment, including chemotherapy, radiation therapy, check point inhibitor therapy, and CAR-T therapy. These characteristics are unique to NK cells and demonstrate why they are indispensable in the development of more effective, less toxic treatment options for patients.
The Therapy
NK101 is an infusion of allogeneic, from a healthy adult, natural killer cells that have been supercharged using our proprietary method. The therapy is given using an intravenous drip that traditionally flows over approximately 3 hours. Patients that are currently enrolled in our study have been deemed terminal or incurable, and qualify under compassionate use for palliative care.
One of NKore's main focuses is to determine the amount of infusions each patient will need based on age, weight, cancer type, and stage of cancer. With a plan to file for FDA approval in late 2025, NKore expects to open up small scale safety trials in blood cancer first, followed by solid tumors in subsequent trials. It is our hope to continue administering our therapy to patients as one off treatments if they meet "compassionate use" qualifications while running larger trials in parallel.