Sophie Ryan
When our cofounder Tracy Ryan’s daughter Sophie was diagnosed with a low-grade brain tumor at 8½ months old, she and her husband, Josh, were devastated. Few advancements had been made in over 40-years for pediatric brain cancers, and the only option for Sophie was toxic chemos that had no chance of curing her disease. It became their mission to find a cure for Sophie, and other patients like her, that didn't have the same toxic outcomes of conventional therapies.
During this journey, Tracy was introduced to Dr. Jewett and learned of her focus on immune function. Dr. Jewett agreed to study Sophie’s immune system to better understand the underlying cause of the disease and assist the clinicians in developing a more effective treatment plan.
Working with Sophie, and many other cancer patients over the years, Dr. Jewett developed unique insights into the functioning of the immune system and the pathways of disease that led her to the development of the activation method for supercharging NK cells. With the benefit of this research, it became clear to Tracy and Josh that Sophie’s best chance for a cure would be an infusion of sNK cells.
The synergy between their two worlds led to the creation of NKore BioTherapeutics to commence the clinical development of the sNK cells to eventually make this therapeutic approach available to all cancer patients.
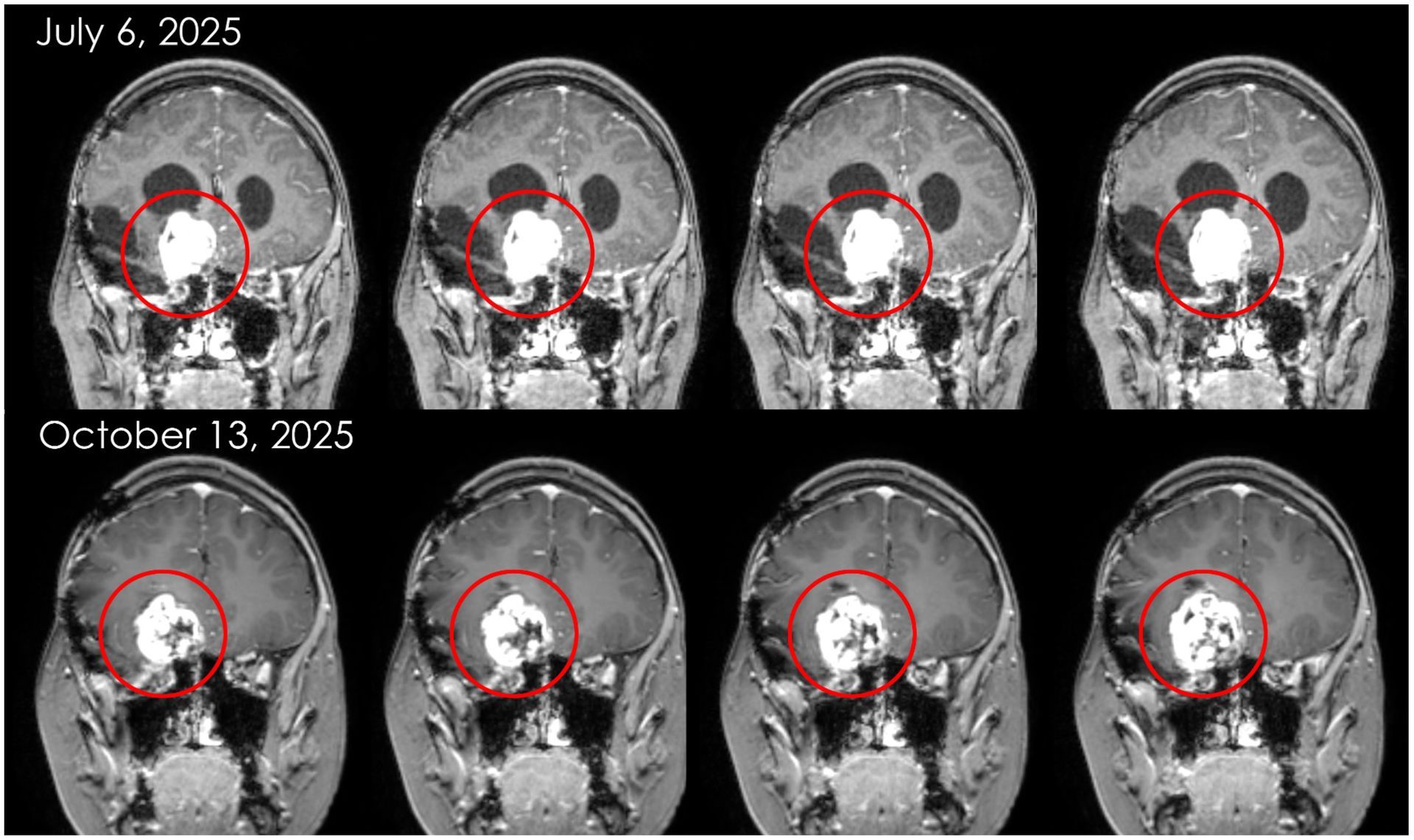
Sophie was the second patient, and first child, to ever receive NKore’s NK101 immunotherapy in December of 2023. She experienced no negative side effects, and her vision improved post-infusion. She received her second infusion in July of 2024 with no negative side effects and early signs of a positive clinical response.